FAQ Orders
We will be glad to answer your questions at: reference-standards@bpt.eurofinseu.com.
Whether you have questions about an offer or an order or want to ask us about a technical problem with the webshop, please do not hesitate to contact us.
If you are not yet a customer:
- Click on the blue “Log in” button on the Eurofins PHAST webshop homepage, scroll down a little if necessary and then click on the blue “Go to registration” button.
- Fill in the webshop registration form (e-mail of the customer = your company e-mail address; e-mail of the invoice recipient = e-mail address of your accounting department) and click on the blue “Submit” button.
- You will receive an e-mail confirming that we have received your registration request. Your Eurofins PHAST webshop access will be set up within 2 working days of your request at the latest.
Why doesn't this happen automatically and immediately?
- As the Reference Standards offer may only be addressed to specialist groups, we are obliged to briefly check the specialist group affiliation of each enquirer. We ask for your understanding.
- Based on the information you provide, we will also collect your company name, registration number, registered office and the name of the managing director. To protect against a possible violation of imposed sanctions, a check is carried out in the directories https://sanctionssearch.ofac.treas.gov/ and https://www.finanz-sanktionsliste.de/fisalis/.
- Once the check has been completed, you will receive an e-mail asking you to confirm the link it contains. This procedure, known as double opt-in, serves to protect you and us against unauthorized access to your webshop account.
- We recommend that you change your password after your first login.
If you are already a customer:
- Click on the blue “Log in” button on the Eurofins PHAST webshop homepage.
- Each time you log in, the system will ask you to enter your e-mail address again.
- Enter the e-mail address that you entered when you registered as a customer.
- The webshop system will then email you a 6-digit code (so-called one-time password).
- Please log in to the webshop with this one-time password.
If you have any questions about the registration process, please send us an e-mail to: reference-standards@bpt.eurofinseu.com
n addition to our reference standard offer in the web store, you can also send us requests for quotations and orders by e-mail to reference-standards@phast.com.
Please note that we can only process your requests for quotations and orders if you provide us with your full address, relevant communication data and VAT identification number.
Please do not send us orders more than once, as we cannot cancel duplicate orders.
You can also order Reference Standards from Eurofins PHAST GmbH via B2B e-commerce solutions. The prerequisite for this is that you invite Eurofins PHAST GmbH to use the procurement portal in the respective portal.
We will then upload a Reference Standards catalog for your company, which you must confirm in order to be able to place orders there.
Send us your request directly via your B2B e-commerce solution:
- SAP® Ariba®: Network ID: AN01400633832
- Proactis (until 2017 HUBWOO): User name/ID: PHAST_REF_1
- SY by Cegedim: User name/ID: DE2236469281
If you have any questions about other possible procurement solutions, please send us an e-mail to reference-standards@bpt.eurofinseu.com
You can select and purchase Reference Standards via the reference standard catalogs on our website www.reference-standards.com.
Alternatively, you can also send an e-mail with an order to reference-standards@bpt.eurofinseu.com. Please do not forget to include the complete signature with your company address and VAT ID number.
If you wish to order via your company's e-procurement system, please contact us so that we can coordinate the details of data communication.
After logging in to the Eurofins PHAST Webshop, select the store area that contains the Reference Standards you require: USP, EDQM or BP.
You can use the search function to search for your Reference Standards, which are then displayed in list form.
- Unit price €: All prices quoted are net prices in EURO currency.
- Shipping costs, an exchange rate surcharge in the case of USP Reference Standards if the USD is quoted above the euro and, where applicable, statutory VAT are added.
- Quantity: The number 1 is displayed here by default. The quantity can be increased or reduced using the small arrow symbols.
- Shopping cart: Click once on the shopping cart to add the selected quantity of Reference Standards to the shopping cart. ATTENTION: Clicking the shopping cart again increases the quantity by the same amount.
- The quantity in the shopping cart is displayed in a small orange circle.
- Now click on the blue button with the shopping cart symbol at the top of the navigation bar. You will now see your shopping cart with the items you have selected. You can also make corrections to the quantity or delete items at this point (and click on “Update shopping cart” if you make changes).
- By clicking in the boxes of the General Terms and Conditions and Price and Revocation Regulations, you accept the regulations of Eurofins PHAST GmbH.
- IMPORTANT: Please enter your order number in the “Internal order number/PO number” field.
- Then click on the blue button “Checkout”.
- By clicking in the boxes of the general terms and conditions and price and revocation regulations, you accept the regulations of Eurofins PHAST GmbH.
- On the left-hand side, you will see your delivery address, which you can still change.
- Below this, you will see the details you entered as your billing address when you registered. By clicking on the small orange circle, you can enter another billing address to be used here.
- On the right-hand side you will find a summary of your order, not yet including shipping costs, the exchange rate surcharge for USP Reference Standards if the USD is quoted above the Euro and, if applicable, the statutory VAT. These amounts are shown in detail in the order confirmation.
- After a final check of your entries, click on the blue “Continue to shipping” button.
If you have placed an item in your shopping cart but have not completed the purchase or have canceled the checkout, you can still complete the purchase as long as the item is still available.
As a reminder, you will receive an e-mail from Eurofins PHAST GmbH after the purchase has been canceled, informing you that you still have an item in your shopping cart and that you can still complete your purchase.
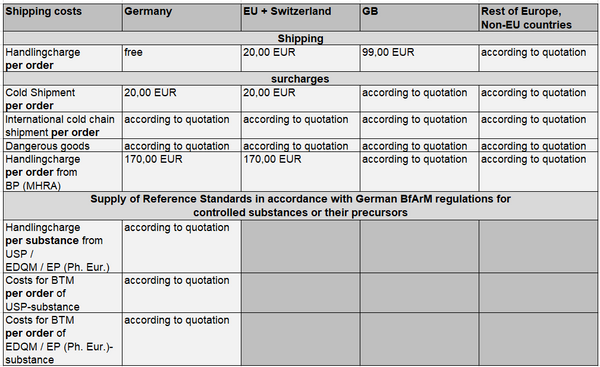
We charge the shipping fees shown in the overview below. In the case of USP Reference Standards to be procured in the USA, we reserve the right to charge an exchange rate surcharge if the euro remains weak against the US dollar.
For further information you may please send us an e-mail to reference-standards@bpt.eurofinseu.com
Eurofins PHAST GmbH delivers according to the globally recognized, uniform contractual and delivery conditions DAP and EXW, which become part of the purchase contract when the order is placed. Within the European Union (EU) and Switzerland, Eurofins PHAST GmbH delivers on the basis of the clause > Delivered AT Place (DAP) <.
Delivered At Place (DAP)
- Delivery / transfer of risk: Eurofins PHAST GmbH makes the items ordered by the customer available at the delivery address agreed with the customer, ready for unloading / acceptance.
- Transport: Eurofins PHAST GmbH organizes and pays a carrier - usually a parcel service provider to the destination / delivery address.
- Transport insurance: The consignment is insured by the parcel service provider as part of the order acceptance, if this is offered. (UPS: Insurance cover of up to € 510 in the event of loss or damage to the shipment; FedEx: Except for € 10 per kg item weight when shipping with FedEx Priority Overnight® Service, FedEx offers neither freight liability insurance nor comprehensive insurance).
- Transportation documents: Eurofins PHAST GmbH will provide both the buyer and the parcel service provider with all freight documents required for transportation.
- Export processing: Eurofins PHAST GmbH handles the entire export process. In third countries - including the United Kingdom (UK) - Eurofins PHAST GmbH delivers on the basis of the clause > Ex Works (EXW) <.
Ex Works (EXW)
- Delivery / transfer of risk: Eurofins PHAST GmbH makes the items ordered by the customer available for unloading / acceptance at the delivery address agreed with the customer.
- Transport: Eurofins PHAST GmbH organizes and pays a carrier - usually a parcel service provider - to the destination / delivery address.
- Transport insurance: The shipment is insured by the parcel service provider when the order is accepted, if this is offered. (UPS: Insurance cover of up to 510 € in the event of loss or damage to the shipment; FedEx: Except for 10 € per kg shipment weight when shipping with FedEx Priority Overnight® Service, FedEx offers neither freight liability nor comprehensive insurance).
- Transportation documents: Eurofins PHAST GmbH will provide both the buyer and the parcel service provider with all shipping documents required for transportation.
- Export handling: Eurofins PHAST GmbH handles the entire export handling. To third countries - including the United Kingdom (UK) - Eurofins PHAST GmbH delivers on the basis of the clause > Ex Works (EXW) <.
We send order confirmations by e-mail to your order e-mail address.
The agreement concluded with Eurofins PHAST GmbH for the delivery of Reference Standards is a sales contract between merchants.
What does this mean?
- By accepting the offer of Eurofins PHAST GmbH or the binding order of Reference Standards, you have concluded a binding purchase contract.
- By accepting the offer or order, German law is deemed to be accepted and agreed. This also applies to our sale of Reference Standards abroad.
- It is no longer possible to change (e.g. article, quantity) or cancel the order after conclusion of the contract. Eurofins PHAST GmbH also excludes the non-acceptance or return of Reference Standards.
- If an order is not accepted or is canceled, Eurofins PHAST GmbH reserves the right to claim damages.
Eurofins PHAST GmbH assumes that the person placing the order has been authorized by his/her employer to place an effective order or to conclude a binding contract. If this requirement is not met at the time the order is placed and the order is contested by the employee's company due to a lack of authorization, the person(s) placing the order may be held liable under German law.
The execution of the delivery of Reference Standards ordered by the customer is always based on the General Terms and Conditions of Eurofins PHAST GmbH, which can be viewed on this website.
As a rule, you will receive an order confirmation by e-mail within one working day.
If you believe that your order may have remained unprocessed, you can contact us directly at reference-standards@phast.com at any time.
Please do NOT send us a new order, as you are liable for all duplicate orders. It is also not possible to cancel a duplicate order.
If the demand for a specific item increases sharply, the supplier of this reference standard may impose a delivery restriction (e.g. 3 vials every 30 days). In this way, the supplier wants to ensure that as many customers as possible can receive at least part of the quantity you require before the stock is sold out or can be replenished.
For you, this means taking into account a longer supply period until you have received the desired quantity of the special reference standard.
If Eurofins PHAST GmbH is currently unable to deliver an ordered article, this may be because the supplier has marked the reference standard in question as “Out of Stock” or “Not in Stock” or the item is “In Stock” but has marked it as “Not Available to Ship”.
In these rare cases, we will inform you immediately. If the expected new availability is already known, you will also receive this information immediately, together with a request as to whether the order should remain valid for this later delivery date. The reference standard will be delivered automatically as soon as it is available again.
If the expected availability is announced at a later date, we will inform you of the re-availability on this new date. You will then also be asked whether the order should be executed on this new delivery date.
If you have any questions about your order or about a specific reference standard, please contact the sales team using the e-mail address: reference-standards@bpt.eurofinseu.com The same applies if you have not found the reference standard you are looking for in the web store.
You are also welcome to contact us by telephone on +49 6841 9242-0. Please also call us if you need immediate support.
As Eurofins PHAST GmbH has certain obligations towards its suppliers, it is possible to supply your customers if
- the purchase contract is concluded directly between your customer and Eurofins PHAST GmbH.
- both the invoice address and the delivery address are within the European Union.
Eurofins PHAST GmbH mainly uses the parcel/logistics service provider UPS for the delivery of your shipments.
UPS offers the option of registering free of charge with UPS My Choice® at https://www.ups.com/de/de/track/ups-my-choice.page. You can track all incoming shipments via a dashboard. You will receive status notifications and updates on the shipments.
As a rule, you will receive your order on the delivery date specified in the order confirmation.
If the order has not arrived within five working days of the advised delivery date, please inform us accordingly - preferably by e-mail to reference-standards@bpt.eurofinseu.com
If you have the tracking number of the parcel/logistics service provider, you can track the shipment status online.
We will try to clarify the definitive status of your shipment and then inform you accordingly.
Our distribution team always tries to avoid partial deliveries. Nevertheless, there are situations where a partial delivery makes sense, e.g. if one of the items ordered is currently not available from the supplier and the waiting time until it is available again would be disproportionately long. A further reason may be the need for special packaging, e.g. if the items in question require refrigeration or are particularly dangerous goods.
If a partial delivery is appropriate, you will be informed accordingly.
Your incoming goods department has detected damage to the reference standard vials or ampoules during the incoming goods inspection.
To make a complaint, please contact our sales team and briefly describe the damage - preferably by e-mail to reference-standards@bpt.eurofinseu.com. To enable us to better assess the damage, please include sufficiently clear photos with your description that clearly show the nature of the defect.
We will then contact you and coordinate the rectification of the defect with you.
Reference Standards cannot be returned.
There are two main reasons for this:
- By accepting the offer from Eurofins PHAST GmbH or placing a binding order for Reference Standards, you have concluded a binding purchase contract.
- Eurofins PHAST GmbH would in any case have to dispose of the Reference Standards returned by you at your expense, as it cannot accept any liability for the storage conditions prevailing at your premises, even if these comply with the regulations.
We ask for your understanding.
The shipping and storage conditions of Reference Standards may differ:
- The storage conditions for an unopened USP reference standard can usually be found on the label of the container, as they may change from one batch to another.
- If there are no specific instructions or restrictions on the reference standard label, the Reference Standards should be stored at room temperature and protected from moisture, light, freezing and excessive heat.
- Once a reference standard has been opened, the manufacturer's warranty for the stability of the contents is void, even if the specified storage conditions continue to be observed.
- Therefore, no manufacturer guarantees the continued usability of Reference Standards that have already been opened. The decision on the continued suitability of opened Reference Standards is the sole responsibility of the user.
- The manufacturer's guarantee for the stability of the contents is also rejected as soon as the Reference Standards are stored under conditions other than those specified by the manufacturer.
- If a reference standard must be refrigerated or shipped under continuously refrigerated conditions on the basis of scientific findings, refrigerated shipping will be carried out accordingly up to the specified delivery address. The recipient is solely responsible for maintaining further refrigeration. For USP Reference Standards, please refer to the USP-NF online publication in the General Chapter <659> Packaging and Storage Requirements for definitions of storage and handling terms.
- For EDQM Reference Standards, please refer to the Ph.Eur. Reference Standards database for storage conditions. The Reference Standards should be stored in such a way that deterioration of the storage conditions is avoided. The Ph.Eur. storage conditions are often more stringent than those described in the monograph (if available).
- The MHRA informs that it ships its BP Reference Standards, which must be stored in a freezer (-20°C), appropriately refrigerated. All other BP Reference Standards are shipped by the MHRA at ambient temperature.
Any unused portions remaining after opening the reference standard container should be stored in accordance with the conditions specified on the label.
However, continued compliance with the specified storage conditions will void the manufacturer's warranty for the stability of the contents of a reference standard once opened. In addition, no manufacturer guarantees the continued usability of Reference Standards that have already been opened. The decision on the continued suitability of opened Reference Standards is the sole responsibility of the user.
The following payment options are available for the purchase of Reference Standards:
- For new or first-time customers, Eurofins PHAST GmbH offers the option of payment in advance. As soon as the accounting department has informed the sales department that payment has been received, the order will be sent to the specified delivery address.
- From the second order onwards, you can switch to purchase on account with the payment term > 14 days net <. From the fifth order onwards, it is possible to agree a different payment term.
To change, add or delete an address, please send an e-mail with a complete signature and your customer number to reference-standards@bpt.eurofinseu.com
We will then carry out the desired address change or deletion.
You will receive confirmation as soon as this has been done.
If you are no longer responsible for purchasing Reference Standards, please send us a short message with your complete signature and customer number to reference-standards@bpt.eurofinseu.com. We will then block your webshop access to prevent unauthorized orders under your name.
We ask your successor to either send us a short e-mail with the new contact details or to register in the webshop.
You will receive Safety Data Sheets, if available, together with the order confirmation by e-mail.
You will receive certificates of analysis, if available, together with the order confirmation by e-mail.
Eurofins PHAST GmbH itself does not issue certificates of analysis (COA).
- USP offers its own USP certificates for a large number of USP Reference Standards (e.g. with molecular information, additional information on use/handling). The PDF files can also be downloaded from the USP homepage.
- The EDQM/Ph. Eur. provides users with a leaflet containing all the information required to perform the tests and examinations described in the relevant monograph(s). This leaflet can also be downloaded from the Ph.Eur. Reference Standards database.
- Users of BP standards can download leaflets with relevant information on analytical data, storage conditions and safe handling instructions, if applicable, from the online catalog. They can be found as a PDF leaflet under “Health And Safety” by clicking on the “Substance Name”.
A reference standard batch is valid and suitable for use as long as it is listed as “current” in the web store.
- If a current batch is exhausted at the USP, it becomes a “previous batch”. At the same time, this USP batch is assigned a date that indicates the maximum valid use. This validity date is usually 3-12 months from the date of exhaustion.
- For the EDQM Reference Standards, with a few exceptions, no expiry date is assigned. Users should only purchase a quantity required for immediate use and must ensure that the EDQM reference standard is valid at the time of use. To do this, they must consult the Batch Validation Statement (BVS) available in the Ph.Eur. Reference Standards database. Once a new batch is officially launched and shipped, the previous batch is usually valid for another six months.
Your personal data is important to us. As part of the supplier-customer relationship, we store personal data for the creation of user accounts and the processing of orders. This also includes technical data such as the IP address or cookies used. The data relevant for shipping is communicated to the contracted logistics service provider and the parcel service.
If legal information requirements apply to a reference standard, authorities will also receive personal data.
Your data will be stored in accordance with the legally prescribed periods.
For further information, please read our data protection information or the data protection declaration on our homepage.
With its two business areas, laboratory services for the development of human and veterinary pharmaceuticals and laboratory supply with the daily analytical requirements for Reference Standards, Eurofins PHAST GmbH makes a significant contribution to increasing patient safety.
To this end, Eurofins PHAST GmbH takes on challenging tasks for its customers in the international pharmaceutical industry with growing success: In addition to efficacy and safety, the quality of a drug is of central importance for the patient. In order to ensure the highest possible quality of medicinal products, there are correspondingly strict legal framework conditions for their development, manufacture and control. All related activities are strictly controlled and comprehensively documented. The resulting costs for research-based pharmaceutical and biotech companies are enormous. This is particularly true for the labor-intensive and time-consuming development of drugs. With its comprehensive services, Eurofins PHAST GmbH is an internationally sought-after service company. High-quality reference materials are required for the qualification of laboratory equipment, the calibration and validation of analytical methods and for quality control in daily routine analysis. In order to save time and costs, pharmaceutical laboratories use pharmacopoeia standards, e.g. USP or EDQM, which have an official status and do not have to be recognized separately by the respective regulatory authorities when used in accordance with the pharmacopoeia. Eurofins PHAST GmbH offers the international pharmaceutical industry USP and EDQM Reference Standards in the form of chemical substances and biological preparations for pharmacopoeia-compliant analyses online in the webshop www.reference-standards.com.
Eurofins PHAST laboratories are part of the Eurofins BioPharma Product Testing laboratory network in Germany. Further information at www.eurofins.de/biopharma
The United States Pharmacopeial Convention - also known as the USP for short - is a non-profit American organization that publishes the pharmacopoeia for the United States and also owns the copyrights to it. The pharmacopoeia is published together with the National Formulary (USP-NF - a collection of quality standards or monographs).
Regardless of the state in which they were manufactured, human and veterinary medicinal products for the US market whose active ingredient is precisely defined in the USP monographs must comply with these USP quality standards, as they have the character of a US federal law.
To ensure that the quality standard for identity, strength and purity required by US law can be achieved in the international development and manufacture of drugs, the USP issues chemical substances and biological preparations whose high purity can be used for reference measurements.
The USP also sets the standards for dietary supplements and food ingredients for the US market and provides the Reference Standards required for production.
The application of the USP quality standards is monitored and enforced in the USA by the US Food and Drug Administration (FDA) and other government agencies.
The European Directorate for the Quality of Medicines & HealthCare (EDQM) is a directorate of the Council of Europe.
All EDQM activities aim to protect human and animal health on the European continent and to contribute to the human right of access to quality medicines and healthcare.
To ensure that the same quality standards can be applied throughout the continent, the EDQM produces and publishes official documentation standards, known as monographs, for the authorization, manufacture and quality control of medicinal products. In addition, the EDQM publishes the necessary physical Reference Standards. Furthermore, the EDQM ensures that the official standards are applied to medicinal products and their ingredients in all signatory states to the Convention of the European Pharmacopoeia (European Pharmacopoeia, Ph. Eur. for short), as the quality requirements for medicinal products and their ingredients are legally binding.
The Medicines and Healthcare Products Regulatory Agency (MHRA) publishes the British Pharmacopoeia and sets the quality standards for pharmaceutical substances and medicines in the UK.
In addition, the British Pharmacopoeia is an integral part of pharmaceutical legislation in the Commonwealth countries and provides technical advice to the European Pharmacopoeia.
The UK remains a member of the European Pharmacopoeia (Ph. Eur.) from January 1, 2021 and will continue to reproduce the text of Ph. Eur. for the convenience of the MHRA's customers (leaving the European Union (EU) on February 1, 2020).
The MHRA publishes the British Pharmacopoeia Chemical Reference Substances (BPCRS) and helps users to demonstrate compliance with the BP monographs.
BPCRS from the UK are considered “non-originating” within the EU under the preferential rules of origin.
You can contact the Reference Standards sales team by telephone on +49 (0) 6841 9242 0.
You also can send us an e-mail with your question to the e-mail address: reference-standards@bpt.eurofinseu.com The Reference Standards sales team will then get in touch with you to answer your question.
